I recently had the chance to try out the gen 2 VScan, since my original one, which replaced my stethoscope 4 years ago, suffered from trauma and is currently in a GE operating room undergoing serious surgery.
GE was kind enough to give me a replacement and have me give their next generation a go, so I thought it would be nice to give a little review for potential buyers.
First of all let me put it in perspective. When GE approached me to try the original VScan in 2011, I thought it was a cool toy, but, as an avid sonographer who tended to favour potent devices as opposed to the smaller laptop-based ones, I didn’t expect to fall for the ‘limited’ VScan. They thought I might.
Turned out we were both right. If I had one of Santa’s elves pushing my ICU’s Aloka Alpha 7 behind me, handing me a clean probe and following me around, plugging and unplugging the awesome but bulky device, I would have no need for a VScan. However, I have yet to hear back from the North Pole, and in its absence, the VScan has been an absolutely awesome and indispensable tool. I’m not joking. My stethoscope has joined my antique medical instruments collection (which includes some really cool ancient metallic tracheostomy tubes btw). I use one of this plastic disposable ones if I really, really want to listen for a wheeze (although I find the degree of expiratory phase prolongation to me a much more sensitive sign of outflow limitation…).
I use the VScan every day, for almost every patient. Bedside ultrasound is an integral part of the physical examination one follow up. It beats guessing.
In my experience, it does the trick about 80% of the time. For more challenging patients, or for a more exhaustive examination, I push that Aloka myself. But when following up patients on the wards especially, having the VScan in my pocket is absolutely essential. Not having it is borderline unethical if I’m dealing with genuinely sick patients.
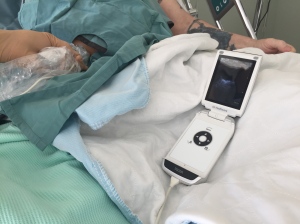
So the new device has a dual probe with vascular and surface capabilities, which is pretty cool, especially if the physician using it has no other device, as vascular exam is really important for procedures and for lung ultrasound (the gen 1 VScan will see B lines but doesn’t have the resolution to look for lung sliding. Here, I’m using the gen 2 on the wards with a sterile sleeve to drain an intra-abdominal hematoma.
So is this a plug for the VScan? Not really. For bedside ultrasound? Absolutely. Every physician taking care of acutely ill patients should have some form of immediately available ultrasound. Anything else is short-changing your patient of the best care they can get.
My favorite article to illustrate the need to integrate bedside ultrasound in daily examination is the following, where novice medical students with ultrasound blow away seasoned, board-certified cardiologists in cardiac diagnosis:
Keep in mind that you can still be a great clinician without bedside ultrasound. But you can be a better one with it.
cheers!
Philippe
p.s. thanks to Mr. Pascal Langlois of GE for the gen 2 VScan loaner!